This article was initially featured on Knowable Magazine.
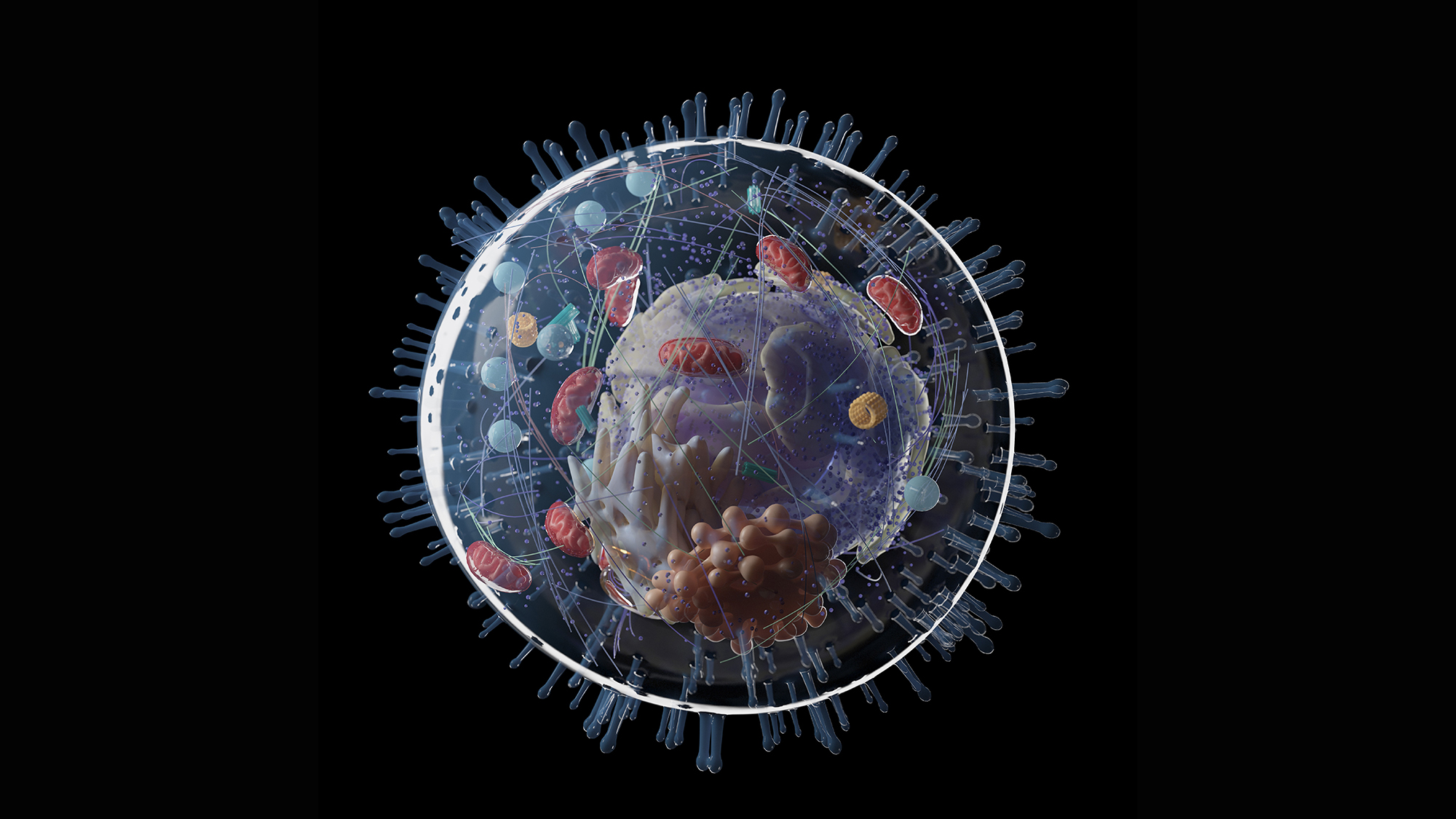
More than 1.5 billion years in the past, a momentous factor occurred: Two small, primitive cells turned one. Perhaps greater than any occasion—barring the origin of life itself—this merger radically modified the course of evolution on our planet.
One cell ended up contained in the other and advanced right into a construction that schoolkids study to check with because the “powerhouse of the cell”: the mitochondrion. This new construction supplied an amazing energetic benefit to its host—a precondition for the later evolution of complex, multicellular life.
But that’s solely a part of the story. The mitochondrion will not be the one vital construction inside complex, eukaryotic cells. There’s the membrane-bound nucleus, safekeeper of the genome. There’s an entire system of inner membranes: the endoplasmic reticulum, the Golgi equipment, lysosomes, peroxisomes and vacuoles—important for making, transporting and recycling proteins and other cargo in and across the cell.
Where did all these buildings come from? With occasions misplaced within the deep previous and few traces to function evolutionary clues, it’s a really powerful query to deal with. Researchers have proposed varied hypotheses, however it’s only just lately, with some new instruments and strategies, that cell biologists have been capable of examine the beginnings of this intricate structure and shed some gentle on its attainable origins.
A microbial merger
The concept that eukaryotes originated from two cells merging dates again greater than 100 years however did not change into accepted or well-known till the Sixties, when the late evolutionary biologist Lynn Margulis articulated her principle of endosymbiosis. The mitochondrion, Margulis mentioned, probably originated from a category of microbes generally known as alphaproteobacteria, a various group that right now contains the bacterium accountable for typhus and one other one vital for the genetic engineering of crops, amongst many others.
Nothing was identified concerning the nature of the unique host cell. Scientists proposed that it already was pretty sophisticated, with quite a lot of membrane buildings inside it. Such a cell would have been able to engulfing and ingesting issues—a sophisticated and energetically costly eukaryotic function referred to as phagocytosis. That may be how the mitochondrion first obtained into the host.
But this concept, referred to as the “mitochondria late” speculation, doesn’t clarify how or why the host cell had change into complex to start with.
In 2016, evolutionary biologist Bill Martin, cell biologist Sven Gould and bioinformatician Sriram Garg, on the University of Dusseldorf in Germany, proposed a really totally different mannequin generally known as the “mitochondria early” speculation. They argued that since no primitive cells right now have any inner membrane buildings, it appears most unlikely {that a} cell would have had these over 1.5 billion years in the past.
Instead, the scientists reasoned, the endomembrane system—the entire hodgepodge of components discovered inside complex cells right now—might have advanced quickly after the alphaproteobacterium took up residence inside a comparatively easy host cell, of a sort from a category referred to as archaea. The membrane buildings would have arisen from bubbles, or vesicles, launched by the mitochondrial ancestor.
Free-living micro organism shed vesicles on a regular basis, for all types of causes, Gould, Garg and Martin be aware, so it appears cheap to suppose they’d proceed to do this when enclosed inside a bunch.
Eventually, these vesicles would have change into specialised for the features that membrane buildings carry out right now inside eukaryotic cells. They would even fuse with the host cell’s membrane, serving to to clarify why the eukaryote plasma membrane comprises lipids with bacterial options.
Vesicles might have served an vital preliminary perform, says biochemist Dave Speijer of the University of Amsterdam. The new endosymbiont would have generated loads of toxic chemical compounds referred to as reactive oxygen species, by oxidizing fatty acids and burning them for vitality. “These destroy everything, they are toxic, especially on the inside of a cell,” Speijer says. Sequestering them inside vesicles would have helped hold the cell protected from hurt, he says.
Another downside created by the brand new visitor might even have been helped by making membranes obstacles, Gould, Garg and Martin add. After the alphaproteobacterium arrived, bits of its DNA would have blended with the genome of the archaeal host, interrupting vital genes. Fixing this might imply evolving equipment to splice out these international items—right now they’re generally known as introns—from the messenger RNA copies of genes, so these protein-making directions wouldn’t be garbled.
But that created yet one more downside. The protein-making equipment—the ribosome—works extraordinarily quick, becoming a member of a number of amino acids collectively per second. In distinction, the intron-removing system of the cell is sluggish, snipping out about one intron per minute. So until the cell might hold the mRNA away from ribosomes till the mRNA was correctly processed, the cell would produce many nonsensical, ineffective proteins.
The membrane surrounding the nucleus supplied a solution. Serving as a spatial barrier, it permits mRNA splicing to complete up within the nucleus earlier than the intron-free mRNA is translated within the cell’s inner fluid, the cytosol. “This is the selective pressure behind the origin of the nucleus,” Martin says. To type it, vesicles secreted by the endosymbiont would have flattened and wrapped across the genome, making a barrier to maintain ribosomes out however nonetheless permitting small molecules to go freely.
An inside-out rationalization
In quick, Gould, Garg and Martin’s speculation explains why endomembrane compartments advanced: to resolve issues created by the brand new visitor. But it doesn’t totally clarify how the alphaproteobacterium obtained contained in the host to start with, says cell biologist Gautam Dey at EMBL in Heidelberg, Germany; it assumes the endosymbiont is already inside. “This is a massive problem,” Dey says.
An different thought, proposed in 2014 by cell biologist Buzz Baum of University College London (with whom Dey as soon as labored) and his cousin, University of Wisconsin evolutionary biologist David Baum, is the “inside-out” mannequin. In this state of affairs, the alphaproteobacterium and the archaeal cell destined to be its eventual host would have lived aspect by aspect for hundreds of thousands of years in an intimate symbiosis, every relying on the other’s metabolic merchandise.
The archaeal cell would have had lengthy protrusions, as seen on some modern-day archaea that reside in shut affiliation with other microbes. The alphaproteobacterium would have nestled up towards these slender extensions.
Eventually, the protrusions would have wrapped across the alphaproteobacterium and enclosed it utterly. But in the course of the lengthy stretch of time earlier than that occurred, the archaeal cell would have begun some spatial division of labor: It would hold information-processing jobs in its heart, the place the genome was, whereas features like protein constructing would happen within the cytosol inside the protrusions.

The energy of the inside-out mannequin, Buzz Baum says, is that it offers the cell eons of time, earlier than the alphaproteobacterium turns into totally enclosed, to evolve methods to control the quantity and dimension of the mitochondrion and other membrane compartments that may finally change into totally inner. “Until you can regulate them, you’re dead,” Buzz Baum says.
The mannequin additionally explains why the nucleus has the form that it does; specifically, it gives a proof for its unusually massive pores. Viewed from inside the middle of an archaeal cell, the lengthy protrusions can be openings that might naturally change into large pores like these, Baum says.
Most vital, the inside-out mannequin explains how the alphaproteobacterium would have gotten contained in the archaeal host within the first place.
Still, the inside-out mannequin has options it wants to clarify. For instance, the mitochondrion would find yourself within the incorrect place—contained in the endoplasmic reticulum, the community of tubes on which sit the cell’s protein-making ribosomes, because the archaeal protrusions wrapped round it. And so an extra step can be required to get the alphaproteobacterium into the cytoplasm.
But Martin’s important objection is that the inside-out mannequin doesn’t present an evolutionary strain that may have prompted the nucleus or other membrane-bound compartments to come up within the first place. The inside-out mannequin “is upside-down and backwards,” Martin says.
The nucleus: A riddle within the center
Though the fashions agree that the mitochondrion advanced from an alphaproteobacterium, they’ve very totally different concepts concerning the origin of the nucleus and other organelles.
In the Gould, Garg and Martin mannequin, the supply for all the buildings would have been vesicles launched by the evolving mitochondrion. Vesicles to include reactive chemical compounds or mobile cargo, and the power to maneuver this cargo round, would have advanced very early. The nucleus would have come later.
In the inside-out mannequin, the nucleus was, basically, the stays of the archaeal cell after it wrapped its membranes across the alphaproteobacterium. So it might have appeared instantly. The endoplasmic reticulum additionally would have fashioned early, created from these squished-together protrusions. Other organelles would have come later—arising, Buzz Baum says, from buds of archaeal membrane.
Thus the fashions additionally make totally different predictions concerning the chemical nature of the membranes of cell organelles—at the least initially—and the way right now’s complex cells got here to have membrane lipids which are all chemically like those in micro organism, not archaea.
In the Gould, Garg and Martin mannequin, to start with all of the membranes aside from the host cell’s outermost one would have been bacterial, just like the membranes of the brand new resident. Then, as bacterial vesicles fused with this archaeal outer membrane, the bacterial lipids would slowly substitute the archaeal ones.
In the inside-out mannequin, the membranes of the nucleus and endoplasmic reticulum — and doubtless others — would have been archaeal, just like the host, to start out. Only in a while, after genes from the bacterial genome moved over to the archaeal genome, would the lipids change into bacterial in nature, Baum suggests.
How to check these concepts? Through experiments, cell biologists are beginning to glimpse methods during which easy vesicles might have diversified into totally different organelles with distinct jobs—by taking over totally different shapes, just like the layered membrane stacks of the trendy endoplasmic reticulum or the Golgi physique, or by ending up with totally different proteins inside them or on their membranes.
They are additionally highlighting the dynamism of the modern-day mitochondrion—and its potential to spawn new membrane buildings.
Take, for instance, the compartment that Speijer thinks advanced early to be able to cope with reactive oxygen species: the peroxisome.
In 2017, cell biologist Heidi McBride of McGill University in Montreal reported that cells missing peroxisomes might generate them from scratch. Working with mutant human fibroblast cells with out peroxisomes, her workforce discovered that these cells put proteins which are important for peroxisome perform into mitochondria as an alternative. Then the mitochondrial membrane launched them as little bubbles, or vesicles.
These vesicles, or proto-peroxisomes, matured into true peroxisomes once they fused with one other sort of vesicle derived from endoplasmic reticulum, which carry a 3rd obligatory peroxisome protein. “It’s a hybrid organelle,” McBride says.
For McBride, this is a sign that peroxisomes—and doubtless other organelles—initially got here from mitochondria (not completely from the endoplasmic reticulum, as beforehand believed). “The presence of mitochondria launched the biogenesis of new organelles,” she says. “In the case of peroxisomes, it’s quite direct.”
Other mitochondrion antics have additionally been famous.
First, a 2021 report from the lab of biochemist Adam Hughes on the University of Utah discovered that when yeast cells are fed poisonous quantities of amino acids, their mitochondria will shed vesicles which are loaded with transporter molecules. The transporters transfer amino acids into the vesicles, the place they received’t poison the mitochondria.
Hughes additionally found that the vesicles shed by the mitochondria can type lengthy, tubule-like extensions with a number of layers, harking back to the layered stacks of the endoplasmic reticulum and the Golgi physique. The buildings persist within the cell for a very long time. “They’re definitely their own unique structure,” Hughes says.
And in 2022, immunologist Lena Pernas, now at UCLA, confirmed that multilayered, mitochondria-derived buildings can type in other contexts, too. When a cell is contaminated by the parasite Toxoplasma, her workforce discovered, the mitochondria encompass the parasite and alter form. The parasite responds, and the upshot is that the mitochondrion finally ends up shedding massive bits of outer membrane.
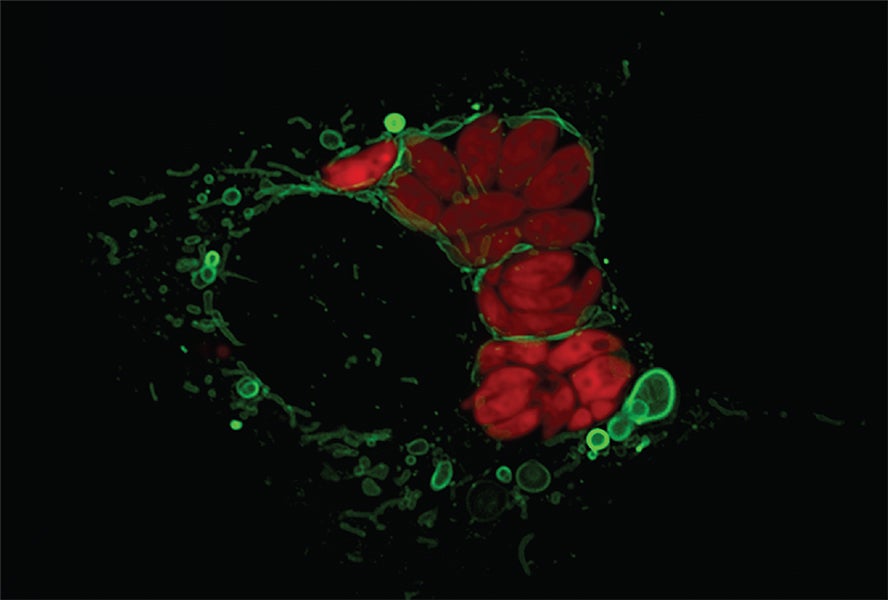
Pernas, who wrote about mitochondrial reworking within the Annual Review of Physiology in 2016, just lately found that these buildings, which initially appear to be easy vesicles, can also develop and tackle extra complex shapes, equivalent to stacks of sheet-like layers. What’s extra, the stress of an infection adjustments what types of proteins are positioned on these shed bits of mitochondrial membrane. Such adjustments open the door for the stacked sheets to behave in numerous methods than they usually would, presenting the chance to tackle new jobs, Pernas says.
The extra Pernas and Hughes examine these buildings—present in fairly totally different cells and situations—the extra related they appear. It’s tantalizing, says Hughes, to think about how a construction like this, forming within the early days of eukaryote evolution, might have advanced over eons of pure choice into a few of the endomembrane compartments present in cells right now.
It might by no means be attainable to know for positive what occurred such a really very long time in the past. But by exploring what can occur in right now’s residing bacterial, archaeal and eukaryotic cells, scientists can get extra readability on what was attainable—and even possible. A cell strikes into one other cell, bringing advantages but in addition issues, setting off a complex cascade. And then, McBride says, “all this stuff blooms and blossoms.”
This article initially appeared in Knowable Magazine, an unbiased journalistic endeavor from Annual Reviews. Sign up for the e-newsletter.