With the discharge of platforms like DALL-E 2 and Midjourney, diffusion generative models have achieved mainstream reputation, owing to their means to generate a collection of absurd, breathtaking, and sometimes meme-worthy pictures from textual content prompts like “teddy bears working on new AI research on the moon in the 1980s.” But a staff of researchers at MIT’s Abdul Latif Jameel Clinic for Machine Learning in Health (Jameel Clinic) thinks there could possibly be extra to diffusion generative models than simply creating surreal pictures — they may speed up the event of recent medication and cut back the chance of adversarial unwanted effects.
A paper introducing this new molecular docking mannequin, known as DiffDock, will probably be offered on the eleventh International Conference on Learning Representations. The mannequin’s distinctive strategy to computational drug design is a paradigm shift from present state-of-the-art instruments that almost all pharmaceutical firms use, presenting a significant alternative for an overhaul of the standard drug growth pipeline.
Drugs sometimes operate by interacting with the proteins that make up our our bodies, or proteins of micro organism and viruses. Molecular docking was developed to achieve perception into these interactions by predicting the atomic 3D coordinates with which a ligand (i.e., drug molecule) and protein might bind collectively.
While molecular docking has led to the profitable identification of medication that now deal with HIV and most cancers, with every drug averaging a decade of growth time and 90 p.c of drug candidates failing pricey scientific trials (most research estimate common drug growth prices to be round $1 billion to over $2 billion per drug), it’s no marvel that researchers are on the lookout for quicker, extra environment friendly methods to sift by way of potential drug molecules.
Currently, most molecular docking instruments used for in-silico drug design take a “sampling and scoring” strategy, looking for a ligand “pose” that most closely fits the protein pocket. This time-consuming course of evaluates numerous completely different poses, then scores them based mostly on how effectively the ligand binds to the protein.
In earlier deep-learning options, molecular docking is handled as a regression drawback. In different phrases, “it assumes that you have a single target that you’re trying to optimize for and there’s a single right answer,” says Gabriele Corso, co-author and second-year MIT PhD pupil in electrical engineering and laptop science who’s an affiliate of the MIT Computer Sciences and Artificial Intelligence Laboratory (CSAIL). “With generative modeling, you assume that there is a distribution of possible answers — this is critical in the presence of uncertainty.”
“Instead of a single prediction as previously, you now allow multiple poses to be predicted, and each one with a different probability,” provides Hannes Stärk, co-author and first-year MIT PhD pupil in electrical engineering and laptop science who’s an affiliate of the MIT Computer Sciences and Artificial Intelligence Laboratory (CSAIL). As a end result, the mannequin does not have to compromise in making an attempt to reach at a single conclusion, which could be a recipe for failure.
To perceive how diffusion generative models work, it’s useful to elucidate them based mostly on image-generating diffusion models. Here, diffusion models progressively add random noise to a 2D picture by way of a collection of steps, destroying the info within the picture till it turns into nothing however grainy static. A neural community is then skilled to get well the unique picture by reversing this noising course of. The mannequin can then generate new information by ranging from a random configuration and iteratively eradicating the noise.
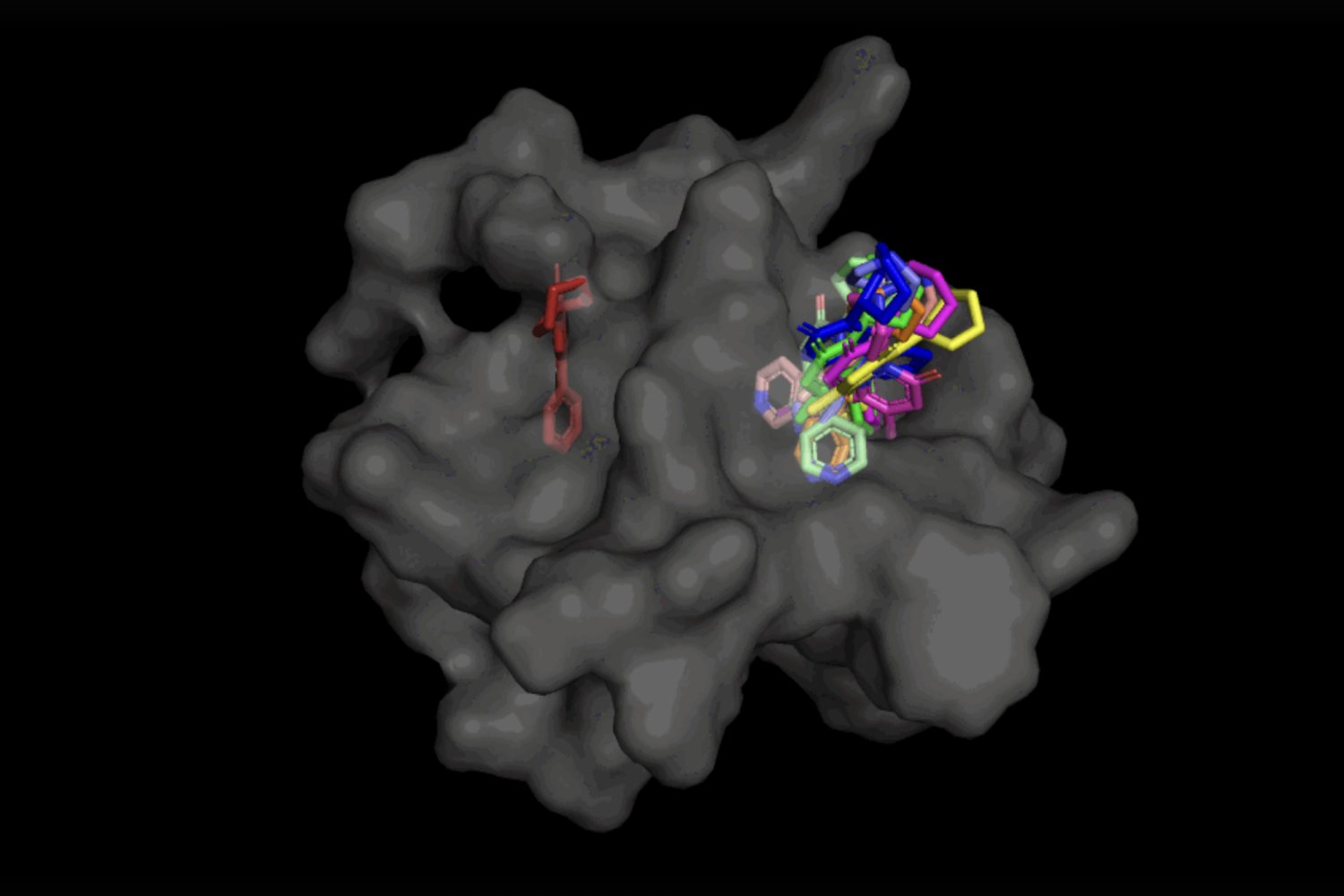
In the case of DiffDock, after being skilled on quite a lot of ligand and protein poses, the mannequin is ready to efficiently determine a number of binding websites on proteins that it has by no means encountered earlier than. Instead of producing new picture information, it generates new 3D coordinates that assist the ligand discover potential angles that may permit it to suit into the protein pocket.
This “blind docking” strategy creates new alternatives to make the most of AlphaFold 2 (2020), DeepMind’s well-known protein folding AI mannequin. Since AlphaFold 1’s preliminary launch in 2018, there was a substantial amount of pleasure within the analysis neighborhood over the potential of AlphaFold’s computationally folded protein constructions to assist determine new drug mechanisms of motion. But state-of-the-art molecular docking instruments have but to exhibit that their efficiency in binding ligands to computationally predicted constructions is any higher than random probability.
Not solely is DiffDock considerably extra correct than earlier approaches to conventional docking benchmarks, because of its means to motive at the next scale and implicitly mannequin among the protein flexibility, DiffDock maintains excessive efficiency, at the same time as different docking models start to fail. In the extra reasonable situation involving using computationally generated unbound protein constructions, DiffDock locations 22 p.c of its predictions inside 2 angstroms (broadly thought-about to be the brink for an correct pose, 1Å corresponds to at least one over 10 billion meters), greater than double different docking models barely hovering over 10 p.c for some and dropping as little as 1.7 p.c.
These enhancements create a brand new panorama of alternatives for organic analysis and drug discovery. For occasion, many medication are discovered by way of a course of referred to as phenotypic screening, by which researchers observe the consequences of a given drug on a illness with out figuring out which proteins the drug is appearing upon. Discovering the mechanism of motion of the drug is then crucial to understanding how the drug may be improved and its potential unwanted effects. This course of, referred to as “reverse screening,” may be extraordinarily difficult and dear, however a mixture of protein folding methods and DiffDock could permit performing a big a part of the method in silico, permitting potential “off-target” unwanted effects to be recognized early on earlier than scientific trials happen.
“DiffDock makes drug target identification much more possible. Before, one had to do laborious and costly experiments (months to years) with each protein to define the drug docking. But now, one can screen many proteins and do the triaging virtually in a day,” Tim Peterson, an assistant professor on the University of Washington St. Louis School of Medicine, says. Peterson used DiffDock to characterize the mechanism of motion of a novel drug candidate treating aging-related illnesses in a latest paper. “There is a very ‘fate loves irony’ aspect that Eroom’s law — that drug discovery takes longer and costs more money each year — is being solved by its namesake Moore’s law — that computers get faster and cheaper each year — using tools such as DiffDock.”
This work was performed by MIT PhD college students Gabriele Corso, Hannes Stärk, and Bowen Jing, and their advisors, Professor Regina Barzilay and Professor Tommi Jaakkola, and was supported by the Machine Learning for Pharmaceutical Discovery and Synthesis consortium, the Jameel Clinic, the DTRA Discovery of Medical Countermeasures Against New and Emerging Threats program, the DARPA Accelerated Molecular Discovery program, the Sanofi Computational Antibody Design grant, and a Department of Energy Computational Science Graduate Fellowship.